Explanation of pharmacological effects of Miglinide calcium in the new version of the medical insurance catalog
Release time:
18 Apr,2020

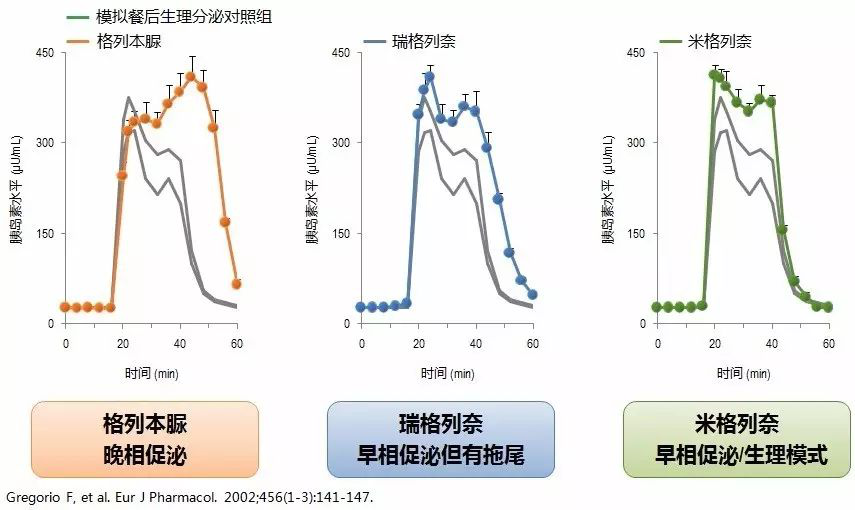
Fig. 1. The proliferation curves of different insulinotropic agents.
Mechanism: All existing insulinotropic agents stimulate endogenous insulin secretion by binding to sulfonylurea receptor 1 (SUR1), a potassium ATP-dependent (KATP) channel of the pancreatic β-cell membrane, which causes closure of the KATP channel. Differences in proinsulin secretion depend on the pharmacokinetic properties of the different drugs, the affinity for binding to SUR1, and the rate of dissociation. Miglinide calcium promotes insulin secretion through two main pathways of action, which are characterized by a "fast on-fast off" pattern (Figure 2). The first pathway is to bind to Kir6.2/SUR1 on pancreatic β-cells, which closes the KATP channel and causes Ca2+ inward flow, leading to vesicle degranulation and insulin release. The second pathway, on the other hand, enters the β-cell and activates the endoplasmic reticulum Ryanodine receptor, which releases Ca2+, leading to vesicle degranulation and insulin release.
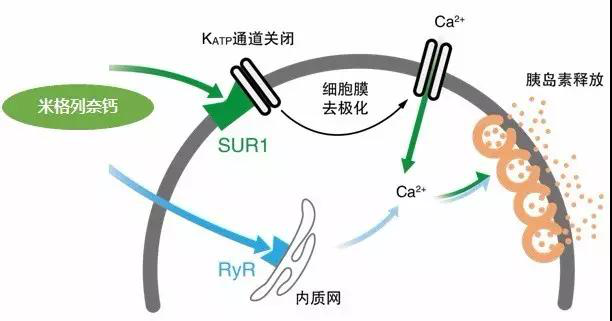
Figure 2. Insulinotropic pathway of action of calcium miglinide.
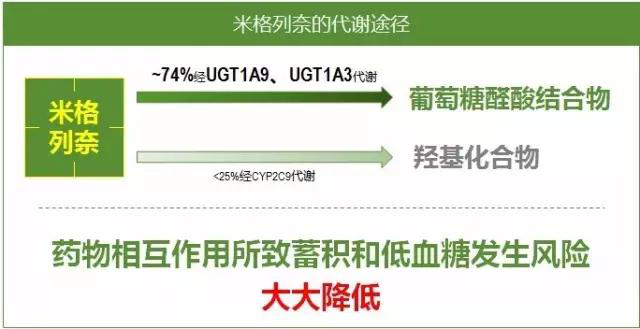
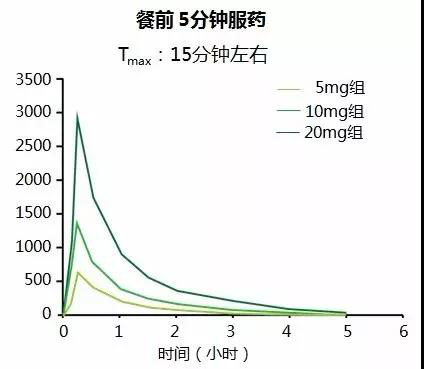
Pharmacokinetics: Miglinide calcium is mainly metabolized by UGT1A9 and UGT1A3, with minimal metabolism by CYP2A9, resulting in fewer interactions with inducers or inhibitors of the P450 family of enzymes, and a greatly reduced risk of accumulation and hypoglycemia due to drug interactions (Figure 3). Miglinide calcium has a rapid peak blood concentration and rapid onset of action. Studies have shown that a single oral dose of mignonide calcium 5 mg, 10 mg, and 20 mg administered to healthy adult males 5 minutes before a meal resulted in maximum blood levels about 15 minutes after administration, with a half-life of about 1.2 hours (Figure 4). With respect to the risk of hypoglycemia occurrence, the risk of hypoglycemia with mignonide calcium was similar to that of placebo.
Treatment with mignonide calcium in patients with newly diagnosed type 2 diabetes mellitus was found to result in greater postprandial glucose reductions, higher rates of HbA1c attainment, and more significant improvements in glycemic fluctuations. A Japanese post-marketing survey showed that the incidence of hypoglycemia in elderly patients (≥65 years of age) treated with mignonide calcium was not significantly different from those <65 years of age. Miglinide calcium did not increase the risk of hypoglycemia in type 2 diabetic patients with comorbid hepatic/renal disease. Miglinide calcium treatment of hemodialysis patients with type 2 diabetes resulted in a 1.1% reduction in HbA1c. Miglinide calcium does not require dose adjustment in patients with CKD stage 3, 4, or renal transplantation; it is used in dialysis patients starting from a low dose of 5 mg before meals.Moreover, it was found that there were no significant changes in hepatic and renal function assessments after miglinide calcium treatment.
Miglinide calcium, as a mealtime glucose regulator, promotes secretion in correlation with blood glucose levels after meals, is easy to take, and the dose can be adjusted according to the therapeutic effect by taking the drug with a meal (orally within 5 minutes before a meal). Given the pharmacological characteristics of Miglinide calcium's binding receptor fast opening and closing, promoting secretion fast starting and falling, restoring the physiological pattern of early-phase insulin secretion, the risk of hypoglycemia is significantly reduced, which is a very good insulin promoter for the control of postprandial blood glucose, especially used in the elderly population and middle-aged people who do not have a regular diet. The inclusion of Miglinide calcium in the updated national medical insurance catalog is a great affirmation of its hypoglycemic effect, and also brings more choices for diabetic patients and clinicians to carry out hypoglycemic treatment.
Other Dynamics
Early-phase insulin secretion defect is a characteristic manifestation of abnormal insulin secretion pattern in type 2 diabetes mellitus patients, and insulinotropic agents have an important role in the treatment of type 2 diabetes mellitus. Coinciding with the successful entry of the new mealtime insulinotropic agent, Miglinide calcium, into the new version of the national health insurance catalog, Diabetes International invited Prof. Mou Yiming of the General Hospital of the Chinese People's Liberation Army to explain the advantages of the application and the applicable population of Miglinide calcium, and to analyze the benefits that will be provided to the patients after its entry into the health insurance.
Defective β-cell insulin secretion is one of the central aspects of the pathogenesis of type 2 diabetes. Coinciding with the successful entry of the new mealtime insulinotropic agent, Miglinide calcium, into the new version of the National Health Insurance Catalog, Diabetes International invited Prof. Zhigang Zhao of the Department of Pharmacy, Beijing Tiantan Hospital, Capital Medical University, to make a professional analysis of the advantages of the action of the new insulinotropic agent from the perspective of pharmacology.
Effective reduction of postprandial hyperglycemia preferred medication - Miglinide calcium capsule
What's worse, the probability of diabetes is increasing among people under the age of 40, as well as pregnant women, and even primary and secondary school students in China.
Golden single product, the heart and brain with treatment - Yixinone dispersible tablets
According to the World Health Organization: the number of people suffering from cardiovascular disease in China has reached 300 million, i.e. 2 out of every 10 adults suffer from cardiovascular disease.
Pioglitazone is the preferred drug for the treatment of type 2 diabetes mellitus.
Diabetes mellitus is a disorder of sugar, fat and protein metabolism caused by a defect in insulin secretion or a decrease in the sensitivity of body tissues and cells to insulin. It is characterized by high blood glucose and is often characterized by excessive urination, drinking, eating and wasting, i.e. "three more and one less".
Drug procurement "national first standard" came drug companies or face different situations
On November 15, the full text of the "4+7" Cities Drug Centralized Purchasing Document was published. With the consent of the Central Committee for Comprehensively Deepening Reform, the State organized a pilot centralized procurement of medicines, and the scope of the pilot area is Beijing, Tianjin, Shanghai, Chongqing, as well as Shenyang, Dalian, Xiamen, Guangzhou, Shenzhen, Chengdu, Xi'an, and other 11 cities (referred to as the "4 + 7 cities"). According to the procurement documents, the first batch of banded procurement catalog totaled 31 varieties, including cefuroxime tablets, escitalopram oxalate tablets, and resuvastatin calcium tablets that have been full of three enterprises through consistency evaluation.



